Our Job Offers
Join us and make a difference for our patients!
We offer you an extraordinary chance to learn, to develop and to be part of an exciting experience and team.
About us
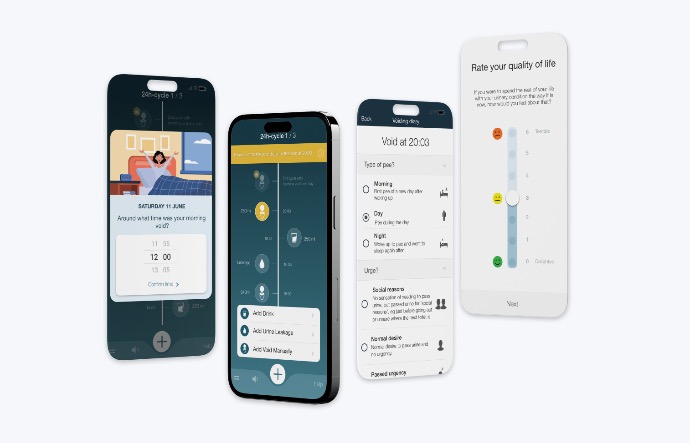
Minze Health is a small yet talented team, that develops impactful medical products for patients suffering from incontinence and other urological conditions. It is our daily mission to improve the quality of life of these patients with our screening and remote monitoring solutions for the patient’s condition.